https://doi.org/10.1140/epjc/s10052-024-13283-7
Regular Article - Theoretical Physics
Thermodynamics of a rotating hadron resonance gas with van der Waals interaction
Department of Physics, Indian Institute of Technology Indore, Simrol, 453552, Indore, India
Received:
22
April
2024
Accepted:
21
August
2024
Published online:
16
September
2024
Studying the thermodynamics of the systems produced in ultra-relativistic heavy-ion collisions is crucial in understanding the QCD phase diagram. Recently, a new avenue has opened regarding the implications of large initial angular momentum and subsequent vorticity in the medium evolution in high-energy collisions. This adds a new type of chemical potential into the partonic and hadronic systems, called the rotational chemical potential. We study the thermodynamics of an interacting hadronic matter under rotation, formed in an ultra-relativistic collision. We introduce attractive and repulsive interactions through the van der Waals equation of state. Thermodynamic properties like the pressure (P), energy density ( ), entropy density (s), trace anomaly (
), entropy density (s), trace anomaly (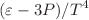 ), specific heat (
), specific heat (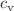 ) and squared speed of sound (
) and squared speed of sound (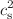 ) are studied as functions of temperature (T) for zero and finite rotation chemical potential. The conserved charge fluctuations, which can be quantified by their respective susceptibilities, are also studied. The rotational (spin) density corresponding to the rotational chemical potential is explored. In addition, we explore the possible liquid–gas phase transition in the hadron gas with van der Waals interaction in the T –
) are studied as functions of temperature (T) for zero and finite rotation chemical potential. The conserved charge fluctuations, which can be quantified by their respective susceptibilities, are also studied. The rotational (spin) density corresponding to the rotational chemical potential is explored. In addition, we explore the possible liquid–gas phase transition in the hadron gas with van der Waals interaction in the T –  phase space.
phase space.
© The Author(s) 2024
 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Funded by SCOAP3.




